Table of Contents
Chemical Safety and Laser Safety
Basic training
All lab users are required to complete OSU's online training modules for basic lab safety. You must go through this process before you are given keys to the lab.
- Ethan will add your name to the lab safety management tool (SciShield) which is administered by OSU's Environmental and Health Safety (EHS) Department.
- SciShield will contact you will a list of training materials. This will include things like hazardous waste awareness, compressed gas safety, sharps safety, eyewash and safety showers and fume hood safety.
- Complete the online training modules. You'll be logged in with onid credentials and the SciShield system will record the trainings.
As a complement to the basic lab safety training, you must also complete this quiz which is specific to the Minot Lab: chem_safety_quiz9.doc. Fill out this quiz and email it to Ethan. Some of the answers are found on this wiki page— scroll down to see information specific to the Minot Lab.
Beyond the basic training
Research projects in our lab often use
- Fume hoods
- Compressed gas cylinders (1-minute YouTube video)
- Lasers
- Cryogenic liquids/gases
- Nanomaterials
- Sharps
- Shop equipment
- Drying oven
If you anticipate exposure to any of this equipment/materials you need to complete additional online training. Please ask Ethan if you are in doubt about which training is required. Follow this process:
- Go to the OSU EHS training home page
- Click on the training needs tool
- In the “Materials” section, select the hazardous materials that you will be working with.
- In the “Equipment” section, select the hazardous equipment that you will be working with.
- At the bottom of the page you will see list of required training modules.
- Complete these online modules. Use your onid username/password to take the quiz or to acknowledge completion.
- You will receive emails verifying completion of each module. Forward these emails to Ethan.
For a comprehensive resource on lab safety, download a free pdf copy of “Prudent Practices in the Laboratory” published by the National Research Council. “Prudent practices” is an excellent resource when you are developing a new standard operating procedure (SOP).
Lab specific notes
The notes below highlight some of the points you learned in OSU online training modules, and how they apply to our labs in 306 and 308 Weniger. These notes are not a substitute for the online training.
Liquid Chemicals and Powdered Substances
- There is one fume hood for organic solvents (acetone, ethanol, IPA) and one fume hood for acids. Never bring the organic solvents into the acid hood. Never bring acids into the organic solvent hood. This absolute/unbreakable rule protects us from the risk of accidentally mixing an oxidizing agent (e.g. nitric acid, see video) with a hydrocarbon fuel (e.g. acetone or IPA).
- Take labeling very seriously. There should never be unlabeled liquid in the lab, even for a second. It is very hard to identify a chemical when it has been forgotten (it only take one distraction to mix up two beakers). This rule protects our devices, protects us from accidentally mixing the wrong chemicals and protects us from treating dangerous chemicals as if they were safe.
- Before using a chemical for the first time, read the MSDS. In the lab there is a folder of MSDS sheets (they are shipped with the chemical, save the MSDS when you open a box!). If the MSDS is not in the lab folder, you must print a copy and add it to the folder (go to the manufacture website or try an online database).
- We use HF (hydrofluroic acid) in Weniger 306. HF is arguably the most dangerous acid used at the university. Anyone wanting to use HF or the acid hood needs additional training. Some useful links include Princeton Uni. EHS, Center for Disease Control
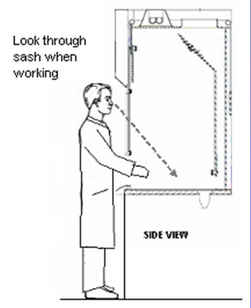
Fume hoods - General info
Solvent Fume Hood
Required protective clothing (minimum):
- Safety goggles
- Nitrile gloves
- Closed-toe shoes
If you get acetone on your nitrile gloves you will need to change gloves. The penetration time is very quick (less than one minute) for acetone, dichloromethane (DCE), toulene and other solvents on thin nitrile gloves. To learn more, google “breakthrough time for nitrile gloves”.
Protocol for disposing solvents: Acetone, Ethanol and Isopropanol are all organic solvents. They are collected together in the waste bottle labeled “organic waste”. When this waste container fills up use the online form to request hazardous waste pickup. Do not dispose by evaporation (i.e. do not leave unsealed containers of solvent in the fume hood).
Chlorinated solvents sometimes require special handling (they are more toxic). The most common one used in our lab is Chloroform, used to make the stamp for 2D Materials Transfer. A small amount of DCE is allowed in the solvent waste container. Check the label of the solvent waste container to know the allowed fraction. For more info contact environmental health and safety.
For more information see the EHS disposal guide.
Working with CNT powder: Our standard protocol is based on Stacey Harper's lab (Prof in nanotoxicology). Inhalation of CNTs is the biggest risk you face (i.e. like asbestos inhalation which causes lung cancer). To weigh CNT powder the scale is first moved inside the fume hood. If needed, a static wipe is rubbed on the scale and the spoon before scooping out powder. The container of CNT powder is never open outside of the fume hood.
Acid fume hood
A practical test must be passed before using the acid fume hood. For the test, you will dip a chip in nitric acid for 30 s. All safety protocols for labeling, pouring, handling, neutralizing and disposing must be followed: Check list for the the acid safety test.
Buddy rule: There are no exceptions to this rule: Never do acid work if you are the only person in the room. You and your buddy must sign the clipboard on the acid hood before you begin work. The buddy can be doing something else, but cannot leave room 306 until you dispose the acid.
Required protective clothing:
- Long pants
- Closed-toe shoes
- Nitrile gloves
- Thicker and longer acid gloves on top of the nitrile gloves. (Watch video of nitric acid on nitrile glove)
- Safety goggles
- Face mask over the safety goggles
- A rubber apron draped over your front
Pause and rehearse: You are about to start a process with more than 10 steps (collecting clean glassware, labeling glass ware, gowning up, pouring acid…). It is critical that you mentally rehearse these steps before you begin, otherwise you will have to gown up and ungown multiple times.
Pouring technique: After pouring acid it is a good idea to clean off the bottle with wet towel. This prevents an unsuspecting person from burning themselves if they touch the bottle.
When diluting acid, add the acid to the water. Do NOT add water to the acid. If water is poured into concentrated acid, it may react very quickly causing the acid to bubble or boil. This may cause acid to be sprayed over the working area. If acid is added to water, the reaction is dispersed, and if there is a violent reaction it will spray water or dilute acid.
If you spill acid on yourself take off your gear and clothes and rinse off. There is an eye wash by the sink on the opposite side of the room of the acid fume hood. It is on a hose and can be pulled out if you need to rinse off your body and not just your eyes. Do not be shy about removing your clothes or getting water on the floor. It is better that getting an acid burn.
Standard cleaning of a bare chip: To clean a silicon chip first set up three beakers, one with pure (fuming) nitric acid, and two with rinse water. It is a good idea to use as little acid as possible to minimize the amount of acid you have to dispose of. Place the chip in the nitric acid and let it sit in the beaker for 1 minute. After a minute has passed remove the chip with a pair of metal tweezers and place the chip in the first rinse beaker. After a short time move the chip to the second beaker, then take it out and blow it dry with nitrogen. We use two rinse beakers because the acid is very strong and the residual acid on the chip is enough to make the water in the first beaker acidic.
Acid waste (waste bottle or neutralize): To dispose of HCl, H2SO4 or HNO3 acids: First check to see if we have a waste bottle labeled with the specific acid. If we have a waste bottle, that is easier to use than neutralizing the acid. If we don't have a waste bottle, neutralize the acid (instructions below) and then pour down the drain.
To neutralize the acid first dilute the acid into a larger volume of water. Then slowly add Sodium Bicarbonate (Baking Soda) until the mixture stops bubbling. We first dilute the acid to make the rate of reaction more predictable. Also make sure to use a relatively large beaker to prevent the solution from bubbling over.
If you used a paper towel throw it away into the acid waste bin under the fume hood. If you feel you got a large amount of acid on the towel you can dunk it in a solution of sodium bicarbonate and water before throwing it away.
What can be poured down the drain?
This list is not all inclusive:
- Sugar solutions
- Dilute non-toxic salt solutions and physiological saline
- Inorganic phosphate or bicarbonate based buffers
- Organic buffers
- Water soluble vitamins
- Surfactants in small amounts
- Neutralized acids and bases (pH between 5.5 - 9.5) with no other hazardous constituents e.g. toxic metals
Hazardous waste pick up
For everything that cannot be poured down the drain, the waste must be collected in waste bottles. There are strict rules about the labeling of waste bottles because many people will be reading and trusting the label (lab mates, university waste handlers, waste contractors who pack and ship waste, the end point facility).
Hazardous Waste Labels and Pickup Requests
All lab members are required to complete the hazardous waste online training and quiz.
Chemical Inventory
Environmental Health and Safety requires us to maintain an inventory of all our chemicals at the following website http://fs-ehs.tss.oregonstate.edu/ehsaweb/ehsawebisapi.dll. The login and password can be found on the group T: drive.
Chemical vapor deposition system
Moldatherm Insulation
- Description: White, chalky ceramic insulator in the CVD.
- Health hazard: Avoid breathing the dust, or contact with skin, eyes, and mouth. Full toxicology is only preliminary, but prolonged respiratory exposure has led to cancer in test animals. At temperatures above 980 C, Moldatherm can partially convert into cristobalite. OSHA limits exposure to cristobalite to 0.05 mg/m^3 of respirable dust. No limit has yet been set for breathing Moldatherm dust.
- Safety precautions: Use gloves whenever operating the CVD or handling the quartz tubing. Do not touch skin or wipe eyes until after washing hands. If heated above 980 C, a respiratory filter is recommended while in use.